On 12th-13th March in Boston, a seminal meeting will take place between the human and veterinary medical industries. The central theme of discussion will be the value proposition to human health companies of out-licensing and research collaborations with veterinary pharmaceutical companies.
At the scientific core of discussions at the Human Biotech and Animal Health Business Partnering Summit is the concept of TCMR, and the evidence that supports the hypothesis that pets make far better models than lab animals for human drug de-risking. With technical and regulatory success rates for drug R&D standing at ~4%, this is a topic of great importance and lively debate.
Several leading human health companies have successfully licensed their technologies into animal health, either maximizing the IP potential of a certain asset by leveraging it in both human and animal health, or getting something out of a dead asset that showed adverse events in humans.
The benefits to both these approaches is non-dilutive revenue – revenue gained without loss of equity – that could potentially reach into the hundreds of millions. While financial payback is less than that of human solutions, operational cost is also much lower in veterinary health. The resulting revenue from animal products has the dual effect of a contribution towards covering the enormous burn rate of an early-stage biotech/pharmaceutical company, and of boosting the company’s appeal to investors looking for revenue-generative companies that have experience with products.
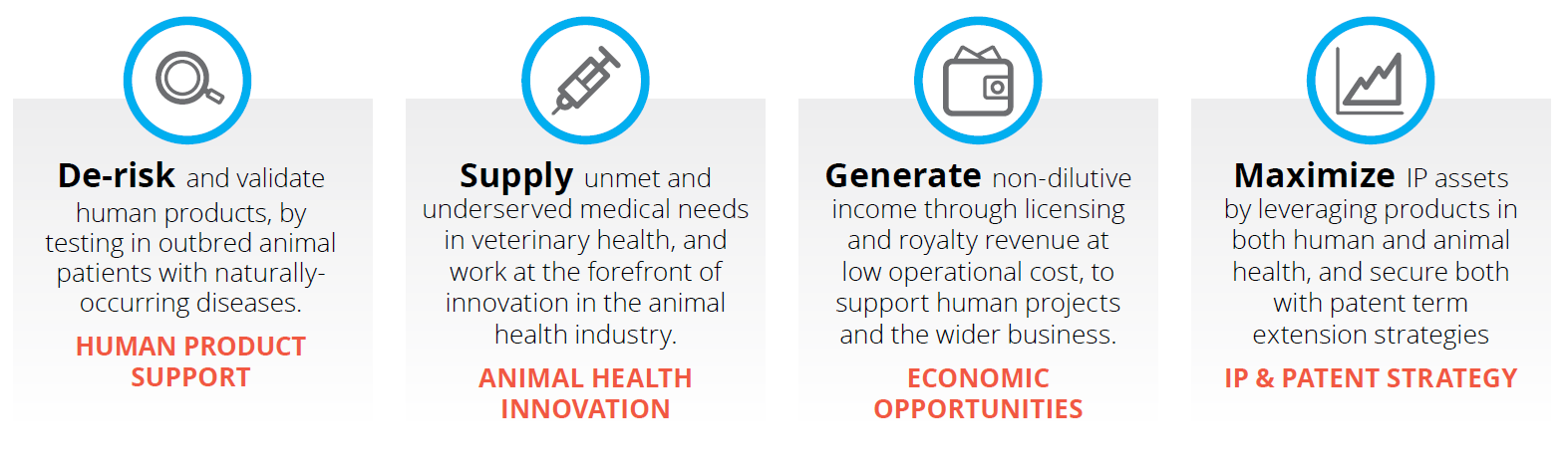 If you have shelved a human drug development project for strategic reasons or because of adverse events in humans, the product may very well be suitable for out-licensing to animal health. You may also have back-up compounds or alternate chemical series which were made as a “safety net” in case the lead compound failed, but were never needed.
If you have shelved a human drug development project for strategic reasons or because of adverse events in humans, the product may very well be suitable for out-licensing to animal health. You may also have back-up compounds or alternate chemical series which were made as a “safety net” in case the lead compound failed, but were never needed.
Many adverse events in humans are inconsequential in animals, like small increases or decreases in blood pressure, suicidal thoughts, impotence, embryo-fetal toxicity or facial flushing, for example. These may make a drug impossible to develop for human use, but acceptable for animals.
Out-licensing does require resources – the conference in Boston will help you understand the type of packages the in-licensing animal health company need, alongside additional information about how regulatory pathways work in the veterinary space, and what unmet medical needs are.
Just how many medical needs are unmet in the veterinary health industry is evidenced by a recent quote by Stephen St. Peter, CEO of Aratana Therapeutics: “About 90 percent of pet medicines are drugs for humans that veterinarians use off-label in dogs and cats.” Indeed, the lack of innovation in the animal health industry due to its small size and the financial pull of the human health industry has led leading veterinary pharmaceutical companies to seek innovation in human medicine.
The modern animal health industry is a far more mature and independent beast now than it was even 10 years ago. Environmental factors, such as the need to find antibiotic alternatives and produce more protein more efficiently, is driving R&D in the ‘production’ animal market, while increased consumer expenditure on pet medicine is doing the same in the ‘companion’ market.
Zoetis, the largest global animal health company, was spun out of Pfizer 5 years ago and is now a global public company with revenue at around $5 billion.
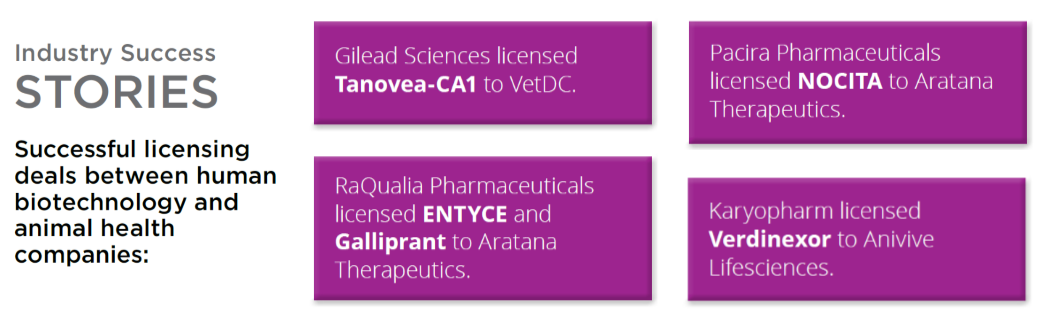 Tapping into this burgeoning industry, several human health companies that have recognized the value of leveraging their products in animal health. Among them, Gilead, Karyopharm, Pacira and RaQualia Pharmaceuticals. Their partners (Aratana Therapeutics and Elanco) are in attendance at the Boston meeting, among representatives from several leading vet health companies. Atsushi Nagahisa, Founder of RaQualia, will join the CEO of Aratana Therapeutics, Stephen St. Peter, to discuss how RaQualia benefited and continue to benefit financially from their out-licensing arrangement.
Tapping into this burgeoning industry, several human health companies that have recognized the value of leveraging their products in animal health. Among them, Gilead, Karyopharm, Pacira and RaQualia Pharmaceuticals. Their partners (Aratana Therapeutics and Elanco) are in attendance at the Boston meeting, among representatives from several leading vet health companies. Atsushi Nagahisa, Founder of RaQualia, will join the CEO of Aratana Therapeutics, Stephen St. Peter, to discuss how RaQualia benefited and continue to benefit financially from their out-licensing arrangement.
Heather Wasserman, Senior Director from Eli Lily, and Christine Adreani, Director from Sanofi Genzyme alongside human biotech CEOs from Cocoon Biotech and Collaborations Pharma to discuss with their vet counterparts how a cross-disciplinary approach could benefit both industries and accelerate medical science.
