Introduction
At Cresset Discovery we endeavour to remain up to date with the changing development landscape, exploring new methods and techniques but also examining trends and problems that are approaching. It was this approach to analysis, and following discussions with our clients, that revealed this research and report from the European Chemicals Agency (ECHA).1
Benefits of the use of fluorinated groups in compound design
The addition of a fluorine atom or atoms to a development candidate regularly has additional benefits other than the often-observed increase in potency. The use of fluorinated groups in design repeatedly improves or solves multiple subtle problems including a compound’s pKa profile, increasing cell permeability, blockage of sites of unwanted metabolism, and general improvement of a compound’s pharmacokinetic profiles. Fluorine has often been successfully used as a general fix-it atom in compound design, solving multiple problems with a single magic bullet.

Figure 1: Title page of the ECHA Restriction Report.
Potential restrictions in use of per- and polyfluoroalkyl containing compounds
The ECHA has identified per- and polyfluoroalkyl substances (PFASs) as an environmental hazard due to their stability and tendency to accumulate in the environment, with potential new legislation to limit their use. Currently plant protection, biocide, human and veterinary medicinal products are exempt from this perspective legislation. However, could this report point to a future direction limiting the use of per- and polyfluoroalkyl substances in new products and a phasing out of existing products? This presents an interesting question: what would happen if per- and polyfluoroalkyl containing bioactive compounds were placed on a restricted chemicals list?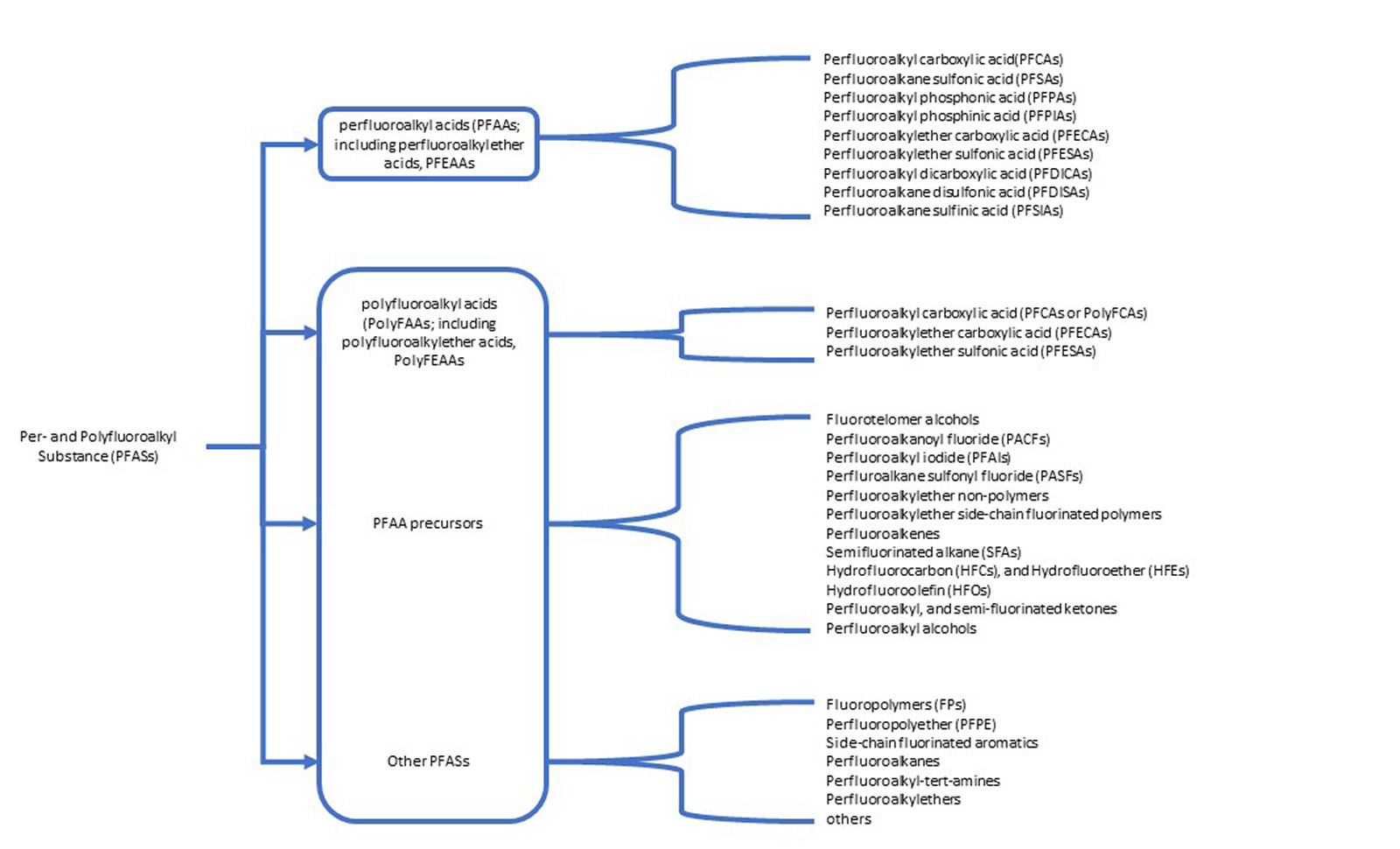
Figure 2: Per- and polyfluoroalkyl substances outlined in the ECHA restriction report.
Obviously, this could not be initiated overnight and would require a phased implementation with time to redesign / replace existing compounds and transformations of compounds in development pipelines, but how would we approach the redesign of existing compounds? At Cresset Discovery we have an established track record of bioisosteric replacement and novel IP development, so we are well placed to consider this (currently) hypothetical problem.
Challenges in redesigning fluorinated compounds
Initially there would be a requirement for the rapid identification of bioisosteric replacements to enable compound analogue redesign without modification of mode of action, and without acceptance of sub-optimum or lower affinity compounds from earlier in the development pathway. Bioisosteric replacements would lead us naturally to using Spark™, our leading bioisosteric replacement tool and de novo design to replace the substructure.2 Virtual screens using either ligand or structure-based methods could also be undertaken to identify new compounds.
If we want to maintain the biological activity and mode of action the question becomes; what is a bioisosteric replacement for per- and polyfluoroalkyl functionality? Currently the answer is there are no standard replacements for these sub-structures, and they tend to perform a unique role in the structures in which they are present. From a medicinal or agrochemical perspective, these types of compounds tend to be termini in the development process. Generally, once positioned favourably in a series, a fluorinated group tends to stay during the development process and is seldom replaced. Experimentally, there is very little information about direct replacements / bioisosteric groups for these systems which maintain activity, ADMET profile, etc.
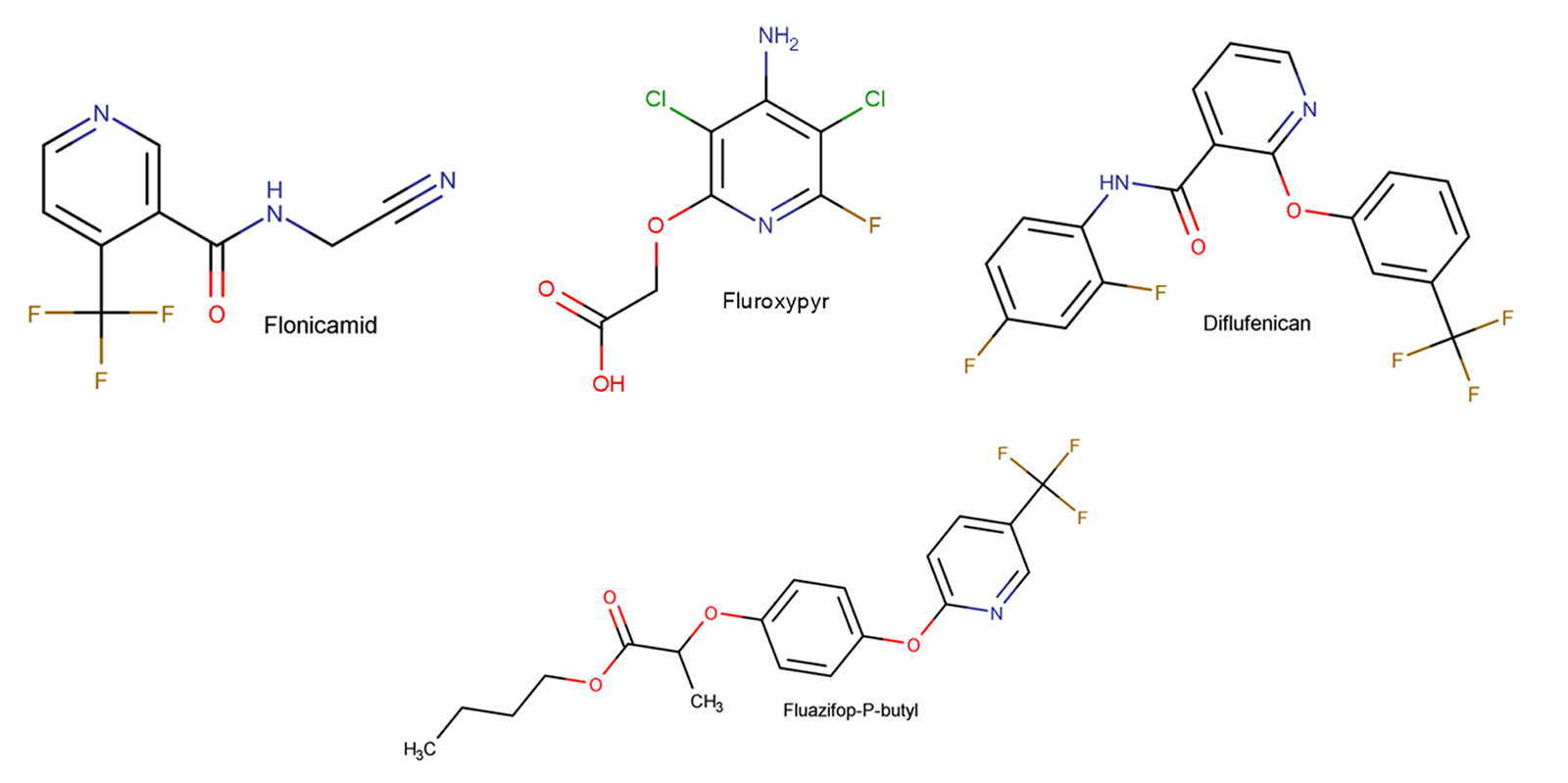
Figure 3: Example of current agrochemicals which would have been affected if per- and polyfluoroalkyl containing bioactive compounds were placed on a restricted chemicals list.
Creative approaches to bioisosteric replacement
If we cannot identify simple like-for-like bioiosteric replacements for per- and polyfluoroalkyl functionalities with direct bioisosteres, or if there are a limited number of acceptable bioisosteres, this could lead to crowded IP space; so more creative solutions must be found. Rather than replacing just the per- and polyfluoroalkyl substructure, replacing a larger fraction of the compound including an attached R-group may be required. This larger substructure replacement offers advantages in that it provides a wider range of substituents. By increasing the size of the fragment to be replaced, this allows for more diversity within the replacement and for different chemotype replacements to be identified for different chemical series, leading to development of a novel IP position for each system under investigation. We know that fluorine atoms have chemical effects both static and electronic, a variety of protein interactions, and AMDET advantages. By increasing the size of the substructure replacement, we increase the chance of identifying bioisosteric analogues which are relevant for the chemical series under investigation.
An example of bioisosteric replacement
As a simple demonstration of this methodology, a bioisosteric replacement calculation was undertaken on tetrafluorocyclcopropyl pyridazine as an exemplar system as shown in Figure 4. As this is only a test case, there are no specific feature requirements taken into account and the results returned are purely unfiltered examples of potential bioiosteres, which may not match all the requirements of a real-life case.
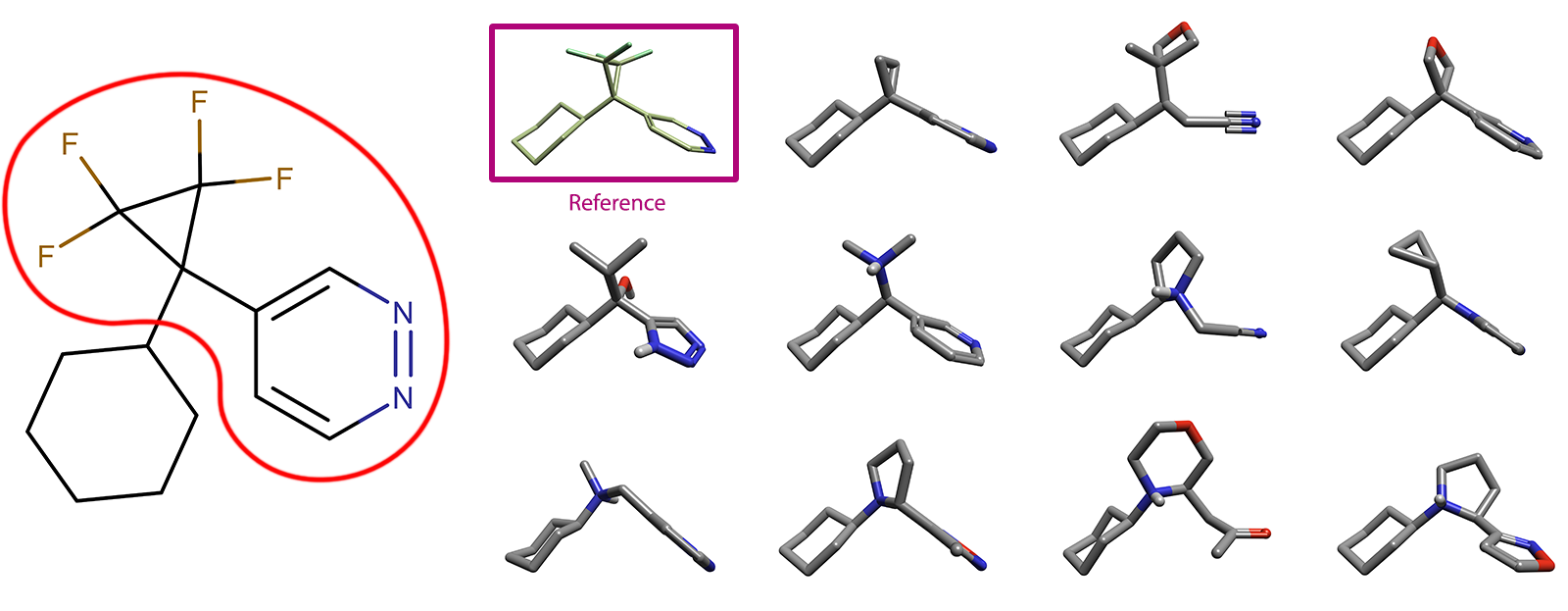
Figure 4: An example of a per- and polyfluoroalkyl bioisosteric replacement and example results.
Generally, replacement of per- and polyfluoroalkyl substances will need to be considered on a case-by-case basis as there are no universally applicable transformations which maintain the wide range of function of these groups. For each case, the biological role of any per- and polyfluoroalkyl component must be evaluated and the bioisosteric replacement tailored to match the structural and biological functionality in the system.
Engage Cresset Discovery to advance your project
Cresset Discovery scientists have the depth of knowledge and experience to approach complex problems like these. Our expertise, combined with access to Cresset’s proprietary technology and specialist software tools, enables us to offer a complete solution to advance your project. Request a confidential discussion to find out how we can add value to your project.
References
- Restriction of per- and polyfluoroalkyl substances (PFAS) under REACH, https://echa.europa.eu/documents/10162/f605d4b5-7c17-7414-8823-b49b9fd43aea
- Spark™, Cresset®, Litlington, Cambridgeshire, UK; https://www.cresset-group.com/spark/; Cheeseright T., Mackey M., Rose S., Vinter, A.; Molecular Field Extrema as Descriptors of Biological Activity: Definition and Validation. J. Chem. Inf. Model. 2006, 46 (2), 665-676