Introduction
Symbiosis Pharmaceutical Services is a contract development and manufacturing organisation (CDMO) specialising in the GMP manufacture and sterile fill/finish of drug products intended for use on clinical trials or commercial supply. Regulatory compliance, technical capability and operational flexibility are at its core.
Manufacturing from a purpose-built FDA-inspected and MHRA-licensed facility, Symbiosis can handle products that require aseptic liquid filling and lyophilisation for a range of complex biologics, viral vectors for use in gene therapies and small molecule drugs. With analytical support, packaging and labelling and QP release, Symbiosis can ensure quality product delivered promptly to the required destination.
Offering fast access to manufacturing slots and accelerated release of drug product, Symbiosis is primed to meet demand for small-scale, fast-turnaround drug product sterile manufacturing.
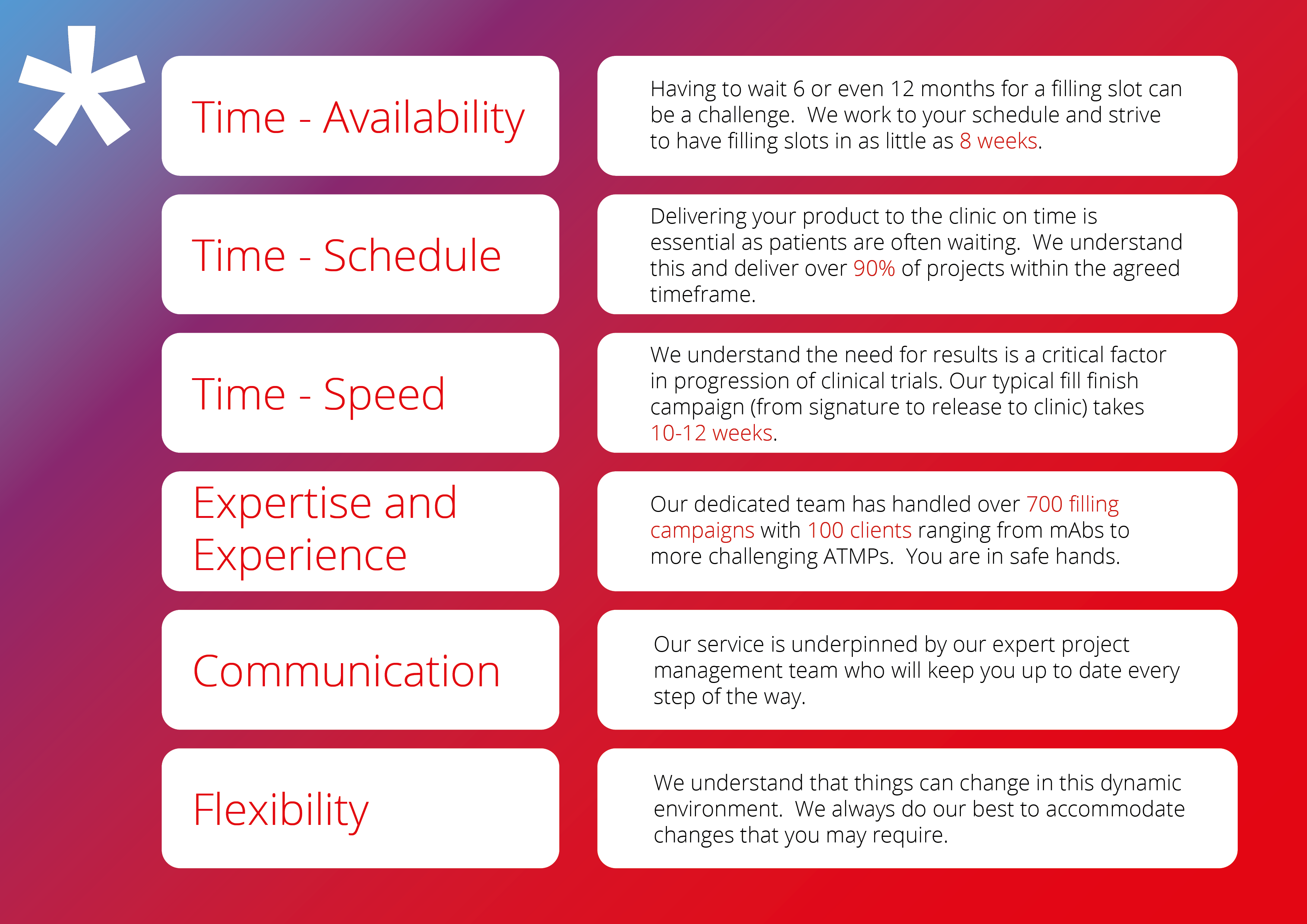
Our USP
In over a decade of operation and significant expansion, Symbiosis has developed a series of value propositions consistent with the demands of sustaining valuable relationships with our partners. We have listened and learned from the feedback and cultivated the following organisational framework that underpins our service offering:
Our reasons for joining Massbio
Symbiosis recognises that Massachusetts is a world epicentre for biotech R&D and as a leading specialist CDMO in aseptic manufacturing, we believe there is a prime opportunity to create meaningful and prosperous symbiotic relationships with the Massbio community. With 50% of our clients based in the US and as we have a US sales office based in Boston, we are keen to develop our network of US based clients. Central to our mission is to forge partnerships and service a community that has an increasing demand for rapid access to GMP manufacturing slots and we intend to continue to grow our market awareness for those organisations that require our services.
Further to this, we know that Massbio is a dynamic and engaged community of life sciences professionals and the opportunity to engage with the industry’s brightest minds will help grow and develop our ability to service this community through the dissemination of sector knowledge and insight. Having the network to develop a novel therapeutic can often be as important as the scientific solution to ensure the successful delivery to the market.
Who we would like to hear from
The core service offered by Symbiosis Pharmaceutical Services is the GMP contract manufacture of sterile pharmaceuticals (Drug Products) for use in clinical trial and low-volume commercial supply:
• Lyophilized
• Liquid
• Cytotoxic
• Biological, including Genetically Modified Organism / Genetically Modified Microorganism viral vector products.
So, if you have a requirement in these areas, then we would love to discuss your requirements.