
The COVID-19 pandemic plunged us all into a global health crisis that redefined what normal life means. The worldwide lockdown forced healthcare systems to re-organize their function to deal with the rising number of coronavirus cases, which had significant impacts on clinical trial operations.
Clinical trial operational plans were affected by the following:
- Significant pressure on health care systems,
- Visitor restrictions at healthcare facilities (for trial participants and sponsor representatives),
- Logistical challenges which impact the completion of trial assessments, subjects’ visits, provision of Investigational Products (IP), etc.
However, we believe that all challenges should be explored as opportunities. One of such opportunities is the potential to perfect remote monitoring of clinical trial sites.
Site monitoring models and associated challenges
Traditional pre-coronavirus models of site monitoring were on-site monitoring, or a combination of on-site and centralized monitoring, where centralized monitoring helped distinguished between reliable and potentially unreliable data and was used to define frequency of on-site monitoring.
Throughout the COVID-19 pandemic, on-site monitoring had to be re-assessed and alternative solutions had to be implemented. Solely centralized monitoring could not address all risks associated with cancellation or reduction of on-site monitoring alone because it doesn’t give proper oversight on how the safety of study participants is assured. It also doesn’t allow proper reception of information about site staff availability, patients’ accessibility, and the peculiarities of IP management at sites.
Remote monitoring principles
In order to address these challenges, CROs can implement remote site monitoring that combines elements of both centralized and on-site activities, utilizing the below principles:
- Have remote monitoring conducted by a dedicated Monitor (CRA) who takes overall responsibility for the site monitoring and site management.
- Conduct Monitoring Events (ME) in a structured manner, where each remote ME contains an interview with the site staff and site documentation review.
- Site documentation review includes general site documentation (like, completed study-specific logs, staff documents updates, updates of the RA and IEC/IRB) and subjects’-specific information review.
- Subjects’-specific information reviewed during remote ME includes the following data:
- Data entries in CRF;
- Reports from IXRS about subjects’ randomization and IP assignment;
- Reports from central laboratories;
- Submitted SAE reports.
A structured interview with the site staff aiming to identify challenges in the study delivery with focus on patients’ safety, compliance with study related procedures and IP management is an essential part of remote monitoring.
In addition to the staff interview the following tools are used:
- Patient safety is assessed based on the CRF entries, reports from the central laboratories and submitted SAE reports (if any).
- Compliance with study procedures is evaluated based on the CRF entries.
- IMP handling gauging and accountability are completed by review of temperature logs, reconciliation IXRS reports and sites IP accountability logs together with subjects’ IP dispensing logs
Strategy that proved to be successful
The implementation of this strategy allows KCR to assure continuity of monitoring processes during the COVID-19 pandemic. All CRAs stay in close contact with the sites, patient safety remains under control and study deliverables at site-level are properly monitored. Additionally, sites are informed about any risks and proposed risk mitigation actions. As a result, even without a physical presence at the site, proper site monitoring is assured.
These experiences with remote monitoring are so successful that instead of being a short-term necessity, we recommend using it as a long-term solution to speed up long overdue changes.
What about SDV?
Some may challenge this approach because remote monitoring does not cover Source Document Verification (SDV) which is traditionally considered to be a crucial activity during on-site monitoring.
There are 2 main purposes of SDV:
- To make sure that all records are Accurate, Legible, Contemporaneous, Original and Attributable (ALCOA).
- To verify if patient data is transcribed correctly from medical source documents to eCRF.
We know that these cannot be verified during remote monitoring, but how important are they really?
Here is a reference to the investigation done by Nicole Sheetz and coauthors which states that 96.3% of data entered by sites is not corrected by any method throughout the duration of the study. From all corrections done, SDV-caused corrections took less than 1/3 of cases.
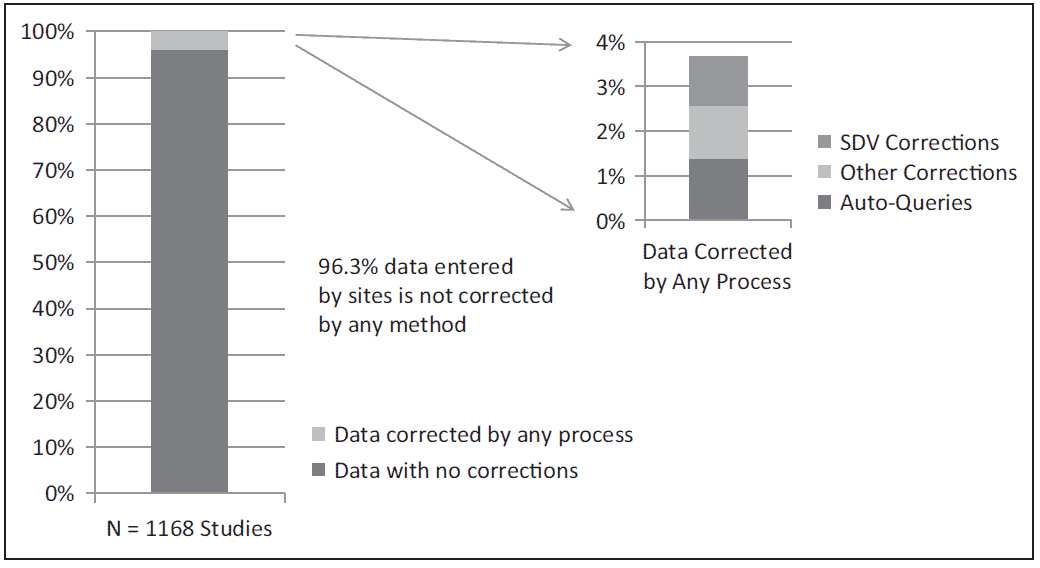
Correspondently, SDV caused data corrections in less than 2% cases.
“In the midst of every crisis, lies great opportunity” – Albert Einstein
Considering all the above, we propose a shift focus from on-site activities to remote monitoring. We do not recommend eliminating on-site visits altogether, but we suggest decreasing their frequency and refocusing on CRAs’ contacts with site staff, source documents review (rather than SDV), and verification of the sites’ facilities.
We also see benefits to move certain site documentation (such as training logs or IP accountability logs) into electronic formats accessible for CRAs at any time point.
This approach makes trial organization more efficient, reduces travel and supports epidemiological safety measures by decreasing face-to-face contacts.
The COVID-19 pandemic has presented a unique opportunity to revise traditional models of site monitoring and implement more freely remote monitoring that will impact the future of clinical research.
About the Author:
Tetyana Byelyayeva, MD
Head of Regional Clinical Operation Services, KCR, Trial Execution
Dr. Tetyana Byelyayeva, MD, PhD manages the Regional Clinical Operations team and leads all learning and development activities for KCR, the international clinical development solutions provider. As the KCR General Manager in Ukraine, she developed the office into one of KCR’s operational hubs. Dr. Byelyayeva is an experienced clinical researcher, teacher and physician with Medical Doctor (MD) and Doctor of Philosophy (PhD) degrees in obstetrics and gynecology.
For more: https://www.kcrcro.com/