
US biotech firms are moving into Europe for all the right reasons, but in some predictably wrong ways. By getting the talent dimensions right up front, they can increase their odds of success.
Some of the most innovative small and midsized US biotech companies are rapidly moving into Europe to expand their market, usually opting to set up direct operations. But often these companies underestimate the difficulties of putting together a Continental team. By failing to account for industry and regulatory differences, product launches can be delayed and targets missed.
For many US biotech companies, Europe can seem a comfortable and logical next step. The region’s cities, landscape, history, and culture are familiar, and expanding into the market seems less formidable than, say, going into emerging markets in Asia. But this familiarity can also conceal traps that can slow and even prevent US companies from reaching their aspirations. While it’s tempting to attribute these missteps to cultural differences, the truth is much more nuanced.
In recent years, dozens of US biotech companies from major hubs including Boston, San Francisco, and Seattle have set up operations in Europe, often focusing on launching one or two core products. One study found that 25 US biotech companies were active in Europe, with direct operations or licensing partnerships, between 2003 and 2013—with most engaging during the last half of that period.[1]
Through discussions with regulatory agencies, organizations, and executives in the region, we have identified a series of pitfalls that should be avoided to gain maximum value from expansion efforts. Some are common to companies in any sector seeking to expand into Europe, while others are unique to the regulatory environment faced by biotech and other pharmaceutical companies.
Recruitment timelines
Recruiting a senior team in Europe takes much longer than it does in the United States. Professional customs can add months to the time needed to bring talented managers on board, and compensation packages must be tailored to meet local expectations.
For example, in Europe the notice period for leaving a position can be as much as six months, compared with the two-week standard for US companies. While departing managers might be given “garden leave” during the notice period—a time in which they collect pay without being required to come to work—they cannot start a new position during this time.
Prospective hires could delay accepting an offer if they risk forfeiting all or part of their year-end bonuses because of the timing of the new offer. Managers at European companies also tend to be more loyal to their employers than are their US counterparts, which can mean longer negotiations. No realistic options are available to accelerate the process significantly, and estimated timelines must take these and similar factors into consideration.
The process can be eased somewhat if the US biotech company is ready with offers that meet European expectations and reflect differences in standard labor practices. For example, Europeans generally expect four to six weeks of vacation compared with the usual two weeks offered in the United States, even if they might not use the whole allotment. Titles and responsibilities should be on par with similar positions at headquarters. Further, sign-up bonuses should take into account any financial losses or career risks linked to leaving an established company.
Appropriate sequencing may also add to the time needed to put together a senior team. Some companies have taken on a functional expert—for instance, in market access or medical affairs—before choosing a head of European operations. While the order might seem trivial compared with the need to build a team quickly, problems can develop if the European head clashes with subordinates or is perceived as having less authority.
In one example, a midsized US biotech company with a promising cancer drug began building a European team once regulatory approval for the market seemed imminent. It searched simultaneously for a European head and heads of human resources and market access. By chance, the European head was the last to be brought on and came into conflict with the other European executives. For the market access leader, the European head wanted an emphasis on launch experience rather than therapeutic oncology, and he had a different talent philosophy than that promoted by the human resources head. Eventually, both the market access and human resources heads had to be replaced, adding more than six months to the planned European launch.
The challenge is exacerbated somewhat because Europeans generally do not accept temporary work contracts, especially if asked to leave a good position in a reputable company. While such contracts might make it easier to rearrange the top team if necessary, labor laws and standard work contracts in Europe make it more difficult and expensive to release an employee than it is in the United States.
Brand recognition can also pose challenges in attracting the best talent immediately. A small or midsized US biotech company may be well known within the domestic talent pool but not among potential European recruits. In some cases, we have found that more than half the candidates approached for senior positions were not familiar with the prospective employer. A well-regarded brand ambassador, such as a local partner or search firm, could help introduce and sell the company to attractive hires.
Location considerations
Location can also have severe implications on a company’s ability to hire the best managers for its European operations. Most European governments try to attract foreign companies with generous tax incentives, but US companies must be careful to consider a wider range of factors when choosing the right location.
One key consideration is superior infrastructure. Easy transportation connections to the rest of Europe and the United States are essential, as are top-rated schools—including international schools for the families of executives coming from outside the country—and a range of amenities, including sports, leisure, and cultural opportunities. And, as with any potential relocation, factors including stable institutions, reasonable tax systems for employees, and low crime rates are crucial.
Further, establishing a European headquarters outside the main biotech hubs—primarily Benelux, Switzerland, and the United Kingdom—can make it more difficult and more expensive to attract the best recruits. ) These centers already have a rich local talent pool and amenities attractive to international transfers. Companies opening offices in other locations will likely have to hire from outside the country to fill their senior teams and face a more difficult sell.
Organization
The organization in Europe will not mirror that in the United States, at least at first. As the European organization develops, it will be relatively small and must be very flexible. In hiring managers, priority should be given to candidates who are able to take on tasks outside their formal mandates, such as managing an affiliate or another function. At least at the beginning, broad-based talent could be vital and save costs.
At the same time, organizations must be able to change course smoothly based on opportunities and successes. For example, instead of rigidly focusing on a single affiliate or market, companies should also be ready to respond if momentum builds unexpectedly somewhere. An emphasis on customer needs, rather than following corporate preconceptions, can help create the needed flexibility and build a business rapidly.
After the head of Europe, it is important to bring talented leaders in market access, regulatory affairs, and medical affairs into the organization. Qualified recruits in these areas are in high demand in Europe, and US companies often underestimate the time needed to identify and court them. Other key positions in the organization—such as marketing, legal, compliance, and HR—are relatively easier to fill and can be hired during the second wave of recruitment (see figure).
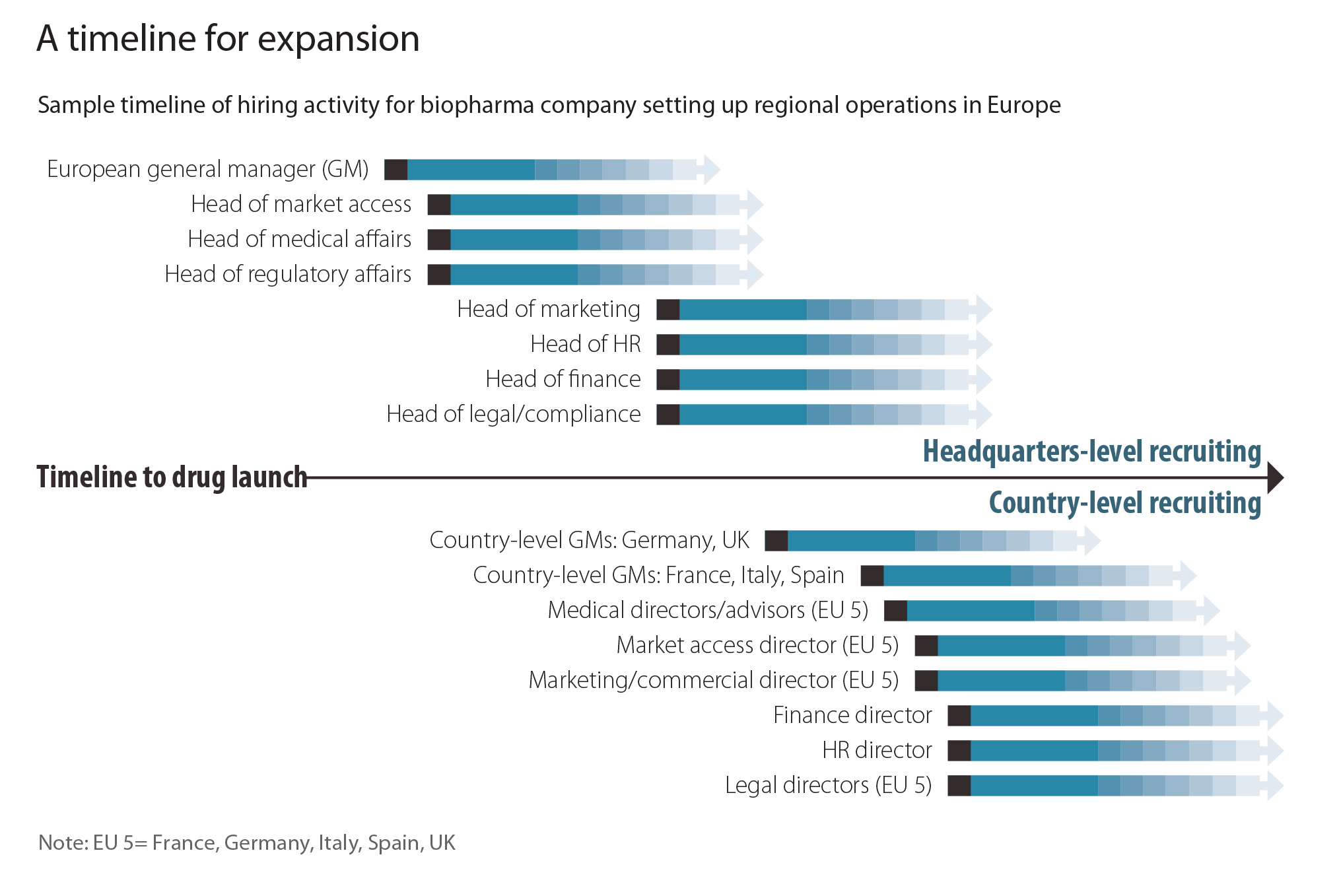
The organization must also present an attractive proposition to potential recruits. For example, promising career paths within the company should be transparent, and companies could also consider stock options and other executive incentives that show a clear commitment. Part of the value proposition could be the company’s products themselves. Often, biotech companies champion what are known as orphan drugs, treatments for diseases that are relatively rare and generally receive little attention from the large pharmaceutical companies. Research in these areas tends to be innovative and provides a clear social benefit, which can be very attractive to young and seasoned executives alike in health industries.
***
In the trepidation and excitement of moving into a new market, crucial considerations are easily overlooked. To bring on the best talent, companies must understand that the process and nuances in Europe can be very different from those followed in building a US organization. Company managers and shareholders must be sure their expectations are realistic, especially the anticipated timelines. Although the similarities between the two markets make moving into Europe very attractive for US biotech companies, neglecting the differences can generate delays, anxieties, and costs.
[1] For a fuller discussion of the two options facing US biotech companies wishing to expand into Europe, see Alain J. Gilbert, Robert J. Easton, and Steven P. Breazzano, “Launch or license: Taking your first drug to Europe,” In Vivo: The Business & Medicine Report, December 2013, bionest.com/publications.
[RM1]From authors
Alan Milinazzo (amilinazzo@heidrick.com), a partner in Heidrick & Struggles’ Boston office, and former CEO of Inspire MD, contributed to this article.
Guest postings on the MassBio blog in no way represent the opinions or endorsement of MassBio or its officers, directors, employees, agents, and consultants. MassBio does not represent or guarantee the truthfulness, accuracy, or reliability of statements or facts posted under the Guest postings on the MassBio blog. MassBio accepts no liability for errors, omissions or representations. The copyright of guest content belongs to the author and any liability with regard to infringement or intellectual property remains with the author.